The other day I poured myself a glass of tap water and then added a few ice cubes. Immediately, the ice cubes started to crack, clearly due to the difference in termperature between the cubes and the water. Did they crack because they were expanding or contracting?
A seasoned whiskey drinker would be the expert here, but my best bet would be that the temperature differential between the surface of the ice cube and the unchanged temperature of the core of the cube probably sets up a heap of internal stresses that are relieved by the cube fracturing. Given that water has a positive temperature coefficient, it would want to expand greatly when it hits the relatively warm water in your glass. This rate of change cannot be absorbed by the varying molecular structure of the cube and so it cracks. Ice cubes can make a squealing sound when dropped into a glass of water. No this is not the cries of agony coming from the cube, but trapped air escaping.
Here you go.
But surely ice contracts as its warms up, no?
No… depending on how hard frozen the ice is… it will EXPAND until it reaches a specific temp (4deg C I think), after which it begins to contract as the liquid sheds off.
I am a thermodynamicist, and have created many programs with Xojo on thermodynamics 
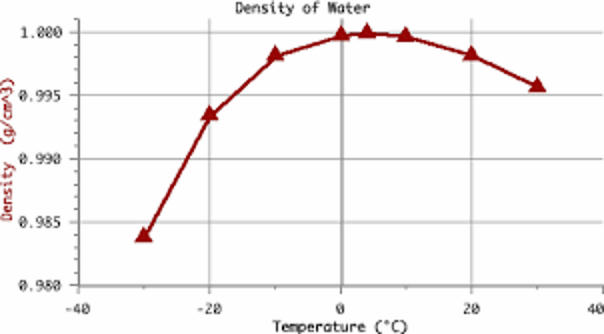
When water is warm and cools, it contracts (shrinks) which increases its density like almost all metals and liquids. Water is rather unique in that when it approaches the freezing point it begins to expand which has a decreasing density. This is why ice cubes float instead of sink.
An ice cube is already cold (below zero Celsius, or 32 F) and placing the ice cube in hot water causes the outer layer of the ice cube to expand (decrease its density) while the inside of the ice cube is contracting (increasing density). This makes the forces in the ice strong enough to cause the ice to fracture.
Sorry, the geek in me came out in this question 
It was the geek in me that asked the question in the first place :). Thanks for the explanation.
“Differential Expansion” is the technical description to what is happening 
As Eugene explained - the centre of the ice cube remains below 0, but the outer part of the ice cube expands due to coming into contact with the warmer water. This causes the outer part of the ice cube to basically be pulled away from its core.
